Firazyr Safety Profile
Indication
FIRAZYR is indicated for the treatment of acute attacks of hereditary angioedema (HAE) in adults 18 years of age and older.1
Warnings and precautions
Given the potential for airway obstruction during acute laryngeal HAE attacks, patients should be advised to seek medical attention in an appropriate healthcare facility immediately, in addition to treatment with FIRAZYR.1
Adverse reactions
The safety of FIRAZYR was evaluated in 3 controlled trials that included 223 patients who received FIRAZYR 30 mg (n=113), placebo (n=75) or comparator (n=38).1
The most commonly reported adverse reactions in patients treated with FIRAZYR were injection-site reactions, which occurred in almost all patients (97%) in clinical trials. These injection-site reactions included bruising, hematoma, burning, erythema, hypoesthesia, irritation, numbness, edema, pain, pressure sensation, pruritus, swelling, urticaria, and warmth. Other common adverse reactions included pyrexia (4%), transaminase increase (4%) and dizziness (3%), as well as rash, nausea, and headache.1
The data described below represent adverse reactions observed from the 2 placebo-controlled trials, consisting of 77 patients who received FIRAZYR at a dose of 30 mg SC, and 75 who received placebo.1
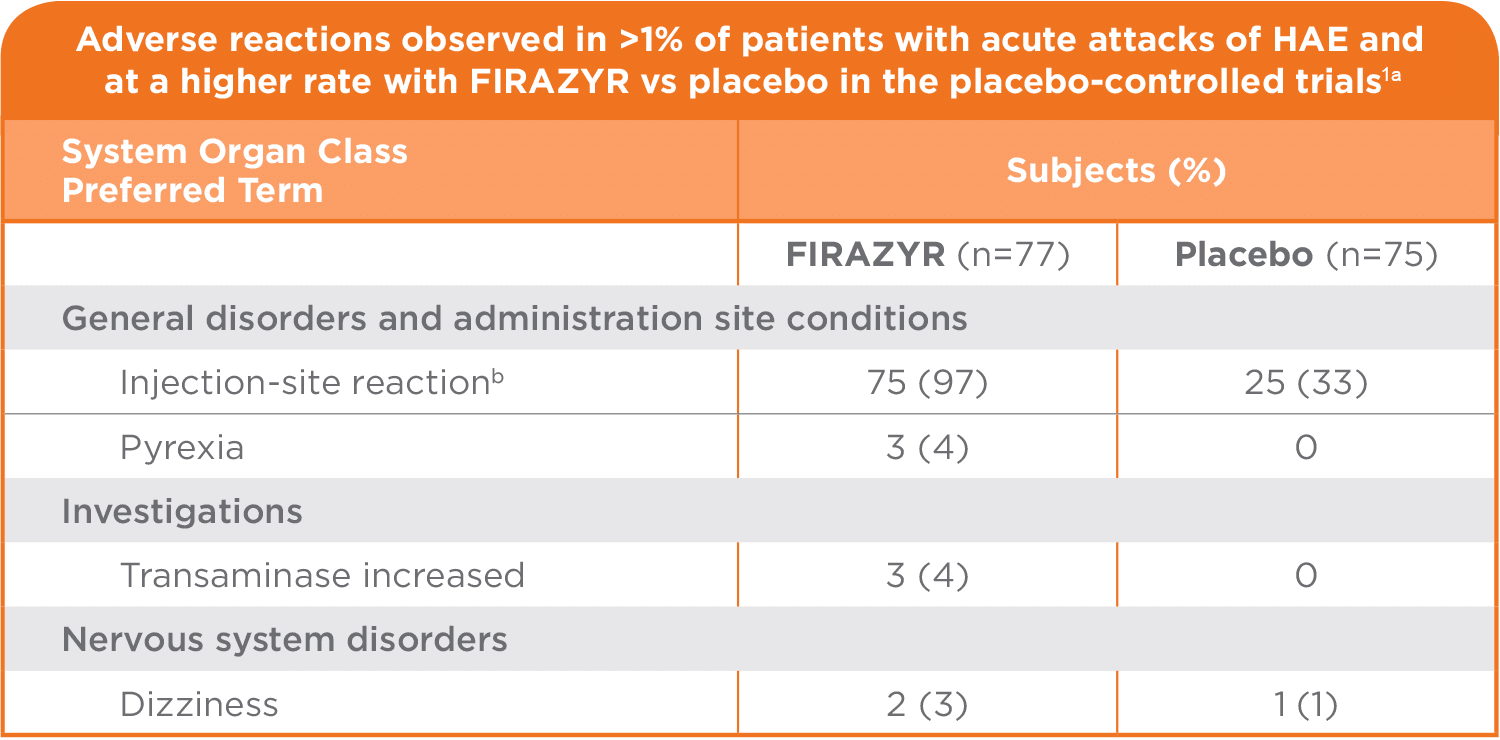
aEvents occurring within 14 days of study drug administration.
bInjection-site bruising, hematoma, burning, erythema, hypoesthesia, irritation, numbness, edema, pain, pressure sensation, pruritus, swelling, urticaria, and warmth.
The third trial was active-controlled and comprised 35 patients who received FIRAZYR 30 mg and 38 patients who received the comparator. Adverse reactions for FIRAZYR were similar in nature and frequency to those reported above.1
Drug interactions
FIRAZYR is a bradykinin B2 receptor antagonist and thereby has the potential to have a pharmacodynamic interaction with ACE inhibitors where FIRAZYR may attenuate the antihypertensive effect of ACE inhibitors. Clinical trials to date have excluded subjects taking ACE inhibitors.1
Use in specific populations
There are no adequate and well-controlled studies of FIRAZYR in pregnant women. FIRAZYR should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.1
Because many drugs are excreted in human milk, caution should be exercised when FIRAZYR is administered to a nursing woman.1
Clinical studies of FIRAZYR included a limited number of subjects aged 65 and over. Elderly patients are likely to have increased systemic exposure. Reported clinical experience has not identified differences in efficacy and safety between elderly and younger patients.1
Safety and effectiveness in patients below 18 years of age have not been established.1
Adverse reactions in self-administration study
The safety of self-administration was evaluated in a separate, open-label trial in 56 patients with HAE. In this trial, the safety profile of FIRAZYR in patients who self-administered FIRAZYR was similar in nature and frequency to that of patients whose therapy was administered by healthcare professionals.1
Immunogenicity
Across repeated treatment in the controlled trials, 4 patients tested positive for anti-icatibant antibodies. Three of these patients had subsequent tests, which were negative. No hypersensitivity or anaphylactic reactions were reported with FIRAZYR. No association between anti-icatibant antibodies and efficacy was observed.1
Please see full Prescribing Information.
To report suspected adverse events, please contact Shire at 1-800-828-2088 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.


