For adults with hereditary angioedema (HAE)
facing the possibility of unexpected attacks...
Take on what's ahead.
Here you will find important information about FIRAZYR, a medicine used to treat acute attacks of hereditary angioedema (HAE) in adults 18 years of age and older. FIRAZYR is the first FDA-approved injection you give yourself under the skin (subcutaneously) to treat acute attacks of HAE—both in and out of the home—after you have been trained by a healthcare professional.
Treat HAE attacks both in and out of the home with FIRAZYR, a portable acute therapy that can go wherever you go
See how FIRAZYR is a proven treatment »Learn the most common side effects associated with FIRAZYR treatment.
Review side effects »Get step-by-step instructions for using FIRAZYR to treat acute HAE attacks upon recognition of symptoms.
Learn how to use FIRAZYR »Find out why treatment experts agree it's important to treat your HAE attacks as soon as symptoms appear.
See why to use FIRAZYR at first sign of an attack »Here to help you throughout your journey with FIRAZYR.
Learn about Takeda Patient Support »FIRAZYR is a portable acute therapy option for adults 18 years of age and older that can go wherever you go, so you are always prepared to treat an attack if needed.
FIRAZYR is supplied as a 3-mL, prefilled, single-use syringe and is self-administered under the skin (subcutaneously) in the abdomen or stomach area.
If your symptoms are not resolved or if symptoms occur again after your first dose of FIRAZYR, additional doses can be administered at least 6 hours apart (no more than 3 doses in any 24-hour period).
FIRAZYR can be used to treat all types of acute HAE attacks, including skin (cutaneous), stomach (abdominal), and throat (laryngeal).

In Trial 1 (n=98), the median time to almost complete symptom relief was 8.0 hours vs 36.0 hours for FIRAZYR and placebo, respectively
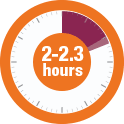
Across 3 controlled clinical trials (n=223), the median time to 50% reduction from baseline symptoms ranged from 2.0 to 2.3 hours with FIRAZYR

In clinical trials (n=225), 9 of 10 attacks were treated with a single dose of FIRAZYR
Across controlled trials (FAST-1, -2 & -3), an analysis showed that the median time to 50% symptom reduction with FIRAZYR was consistent for up to 5 separate non-throat attacks.

FIRAZYR is used to treat acute attacks of hereditary angioedema (HAE) in adults 18 years of age and older.
The most common side effects of FIRAZYR include:
In a self-administration study, 56 people with HAE treated themselves with FIRAZYR. The safety profile of FIRAZYR was about the same, regardless of whether people gave the injection themselves or had it done by a healthcare professional.
Tiredness, drowsiness and dizziness have been reported following the use of FIRAZYR. If any of these occur, do not drive a car, use machinery or do anything that needs you to be alert.
In clinical trials, no hypersensitivity or anaphylactic reactions were reported.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. For more information about FIRAZYR, ask your healthcare provider. For further information, please see the full Prescribing Information.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
FIRAZYR is used to treat acute attacks of hereditary angioedema (HAE) in adults 18 years of age and older. Your healthcare provider will prescribe FIRAZYR for you, tell you when to use it, and teach you or a caregiver how to inject FIRAZYR. Click here for instructions on how to administer FIRAZYR.
If your symptoms are not resolved or if symptoms occur again after your first dose of FIRAZYR, additional doses can be administered at least 6 hours apart (no more than 3 doses in a 24-hour period).
With FIRAZYR, you can inject yourself as soon as you recognize the symptoms of an acute HAE attack after training under the guidance of a healthcare professional
FIRAZYR is used to treat acute attacks of hereditary angioedema (HAE) in adults 18 years of age and older. After starting treatment with FIRAZYR, knowing when—and why—to treat your HAE attacks can help you stay on track with the treatment plan you’ve discussed with your doctor. Here’s some helpful information to keep in mind.

People with HAE, including those taking a preventive therapy, are strongly encouraged to have enough acute treatment for 2 attacks, or at least 2 doses, on hand to be prepared if an attack happens.
Laryngeal attacks can become life threatening. If you have an HAE attack of the throat (laryngeal attack), inject FIRAZYR and then go to the nearest hospital emergency room right away.

Because HAE attacks are unpredictable, always be ready to treat.

FIRAZYR is supplied in a 3-mL, prefilled, single-use syringe and is self-administered under the skin (subcutaneously) in the abdomen or stomach area, after training by a healthcare professional.
With FIRAZYR, you can inject yourself as soon as you recognize the symptoms of an acute HAE attack.



Takeda Patient Support offers tailored support for FIRAZYR.
We understand that living with hereditary angioedema looks different for everyone. We get to know you, understand who you are, and learn what’s important to you—so we can provide the treatment support you need.
Our long-term commitment to the HAE community allows us to better understand and meet your unique needs.
After you and your physician choose a treatment path, Takeda Patient Support is here for you with a range of personalized services.
Takeda Patient Support is a product support program for people who have been prescribed FIRAZYR. Our support specialists are here to address your questions and help get you the resources you need. Some of the resources we offer include:
To learn more about Takeda Patient Support visit: www.takedapatientsupport.com
*To be eligible, you must be enrolled in Takeda Patient Support and have commercial insurance. Other terms and conditions apply. Call us for more details.
Our support specialists are never more than a tap or call away—1‑866‑888‑0660, Monday through Friday, 8:30 AM to 8 PM ET. Not enrolled? You can join Takeda Patient Support in a few simple steps at TakedaPatientSupport.com. If English is not your preferred language, let us know. We may be able to assist you in the language of your choosing.
The program can cover up to 100% of your out-of-pocket co-pay costs, if you’re eligible. To be eligible for this program, you must:
If you can’t afford your treatment, we may be able to connect you to programs that may help.
*IMPORTANT NOTICE: Takeda’s Co-pay Assistance Program ("the Program") provides financial support for commercially insured patients who qualify for the Program. Participation in the Program and provision of financial support is subject to all Program terms and conditions, including but not limited to eligibility requirements, the Program maximum benefit per claim and the annual calendar year Program maximum (“Annual Program Maximum”). The Annual Program Maximum for your prescribed Takeda product can be found by visiting: https://www.takedapatientsupport.com/copay.
By enrolling in the Program, you agree that the Program is intended solely for the benefit of you—not health plans and/or their partners. Further, you agree to comply with all applicable requirements of your health plan. The Program cannot be used if the patient is a beneficiary of, or any part of the prescription is covered by: 1) any federal, state, or government-funded healthcare program (Medicare, Medicare Advantage, Medicaid, TRICARE, etc.), including a state pharmaceutical assistance program (the Federal Employees Health Benefit (FEHB) Program is not a government-funded healthcare program for the purpose of this offer), 2) the Medicare Prescription Drug Program (Part D), or if the patient is currently in the coverage gap, or 3) insurance that is paying the entire cost of the prescription. No claim for reimbursement of the out-of-pocket expense amount covered by the Program shall be submitted to any third-party payer, whether public or private.
Some health plans have established programs referred to as ‘co-pay maximizer’ programs. A co-pay maximizer program is one in which the amount of a patient’s out-of-pocket costs is adjusted to reflect the availability of support offered by a manufacturer’s co-pay assistance program. If you are enrolled in a co-pay maximizer program, your Annual Program Maximum may vary over time to ensure the program funds are used for your benefit (for the benefit of the patient). Takeda also reserves the right to reduce or eliminate the co-pay assistance available to patients enrolled in an insurance plan that utilizes a co-pay maximizer program.
If you learn your health plan has implemented a co-pay maximizer program, you agree to notify the Program immediately. It may be possible that you are unaware whether you are subject to a co-pay maximizer program when you enroll or re-enroll in the Program. Takeda will monitor program utilization data and reserves the right to discontinue assistance under the Program at any time if Takeda determines that you are subject to a co-pay maximizer, or similar program.
The Program only applies in the United States, including Puerto Rico and other U.S. territories, and does not apply where prohibited by law, taxed, or restricted. This does not constitute health insurance. Void where use is prohibited by your insurance provider. If your insurance situation changes you must notify the Program immediately. Coverage of certain administration charges will not apply for patients residing in states where it is prohibited by law.
This Program offer is not transferable and is limited to one offer per person and may not be combined with any other coupon, discount, prescription savings card, rebate, free trial, patient assistance, co-pay maximizer, alternative funding program, co-pay accumulator, or other offer, including those from third parties and companies that help insurers or health plan manage costs. Not valid if reproduced.
By utilizing the Program, you hereby accept and agree to abide by these terms and conditions. Any individual or entity who enrolls or assists in the enrollment of a patient in the Program represents that the patient meets the eligibility criteria and other requirements described herein. You must meet the Program eligibility requirements every time you use the Program. Takeda reserves the right to rescind, revoke, or amend the Program at any time without notice, and other terms and conditions may apply.